— “Unsupported Price Increase” final report based on drugs identified through SB-17, the California drug price transparency law —
— Increases in spending for many drugs under the SB-17 law may be primarily due to increased volume rather than increases in net price —
BOSTON, October 20, 2022 – The Institute for Clinical and Economic Review (ICER) today posted a Final Report on Unsupported Price Increases (UPI) of prescription drugs in California during 2020. This California report used the same methodology for evaluating evidence as that used in ICER’s annual unsupported price increase report at the national level.
“Prescription drugs continue to arrive in the US with increasingly high launch prices that are frequently far above a fair alignment with the demonstrated improvement in patients’ lives,” said David Rind, MD, ICER’s Chief Medical Officer. “Additionally, we very frequently see year-over-year price increases despite a lack of substantial supporting clinical evidence. We recently received a new grant to develop an unsupported price increase report specific to California. The state’s SB-17 report looks at the most frequently prescribed drugs, the costliest drugs by total annual plan spending, and the drugs with the highest year-over-year increase in total annual plan spending in California. We found that, because information on volume and changes in net price in California are not available, the increase in spending for many drugs may be primarily, or even entirely, due to increased volume rather than increases in net price. Thus, drugs with increased utilization can be listed by the state of California even without significant increases in list or net pricing.”
Methodology and Key Findings
In 2017, California passed SB-17, a drug transparency law requiring health plans and manufacturers to report information related to the costs of covered prescription drugs. Consistent with the protocol we released in March 2022, ICER leveraged the annual SB-17 drug lists of brand and specialty drugs with the most significant year-over-year spending increases in California to evaluate whether new evidence has been presented that could justify a price increase.
ICER’s review started with the top 25 specialty drugs and top 25 branded drugs from the SB-17 lists and removed any drug that was determined to have had a national price increase net of discounts less than 2% above the rate of medical inflation. ICER then created a list of 10 specialty and brand drugs with the most significant year-over-year spending increases. Manufacturer feedback on net price increases eliminated the majority of drugs from this list, leaving 3 drugs for review. Manufacturers of these drugs had the opportunity to provide evidence that could justify price increases.
The table below shows the results of the evidence assessments for the three drugs included in the report. All three were judged to have price increases unsupported by new clinical evidence.
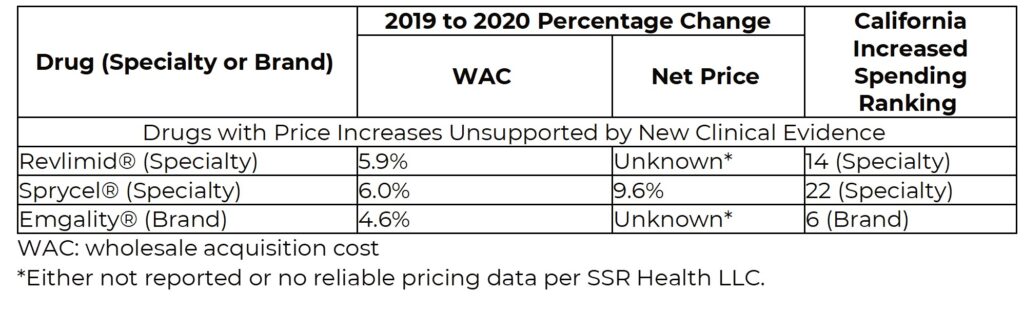
California’s drug price transparency laws are an important first step in examining price increases. Under SB-17, however, the year-over-year changes in net drug prices in California are not reported. Laws focusing on increases in total drug spending risk identifying drugs without significant price increases that are being prescribed more often due to many possible factors. States seeking to identify drugs for which increases in spending may be inappropriate should incorporate a requirement for reporting of net price increases at the state level across all payers.
Earlier this year, ICER received a new grant from the California Health Care Foundation (CHCF) to develop 1) two annual unsupported price increase reports specific to California, and 2) a policymaker guide outlining how to use comparative effectiveness research to ensure that patients have fair access to fairly priced drugs.
About ICER
The Institute for Clinical and Economic Review (ICER) is an independent non-profit research institute that produces reports analyzing the evidence on the effectiveness and value of drugs and other medical services. ICER’s reports include evidence-based calculations of prices for new drugs that accurately reflect the degree of improvement expected in long-term patient outcomes, while also highlighting price levels that might contribute to unaffordable short-term cost growth for the overall health care system.
