ICER’s Impact
In the US, consequential decisions around prescription drug pricing and patient access have historically been made based on limited evidence and without patients in the room. In confidential negotiations, drugmakers and insurers make financial tradeoffs that seriously affect the health and wealth of all Americans. Too often, drugmakers charge as much as they can, insurers react by restricting care and raising copays, and patients are none the wiser.
We use comparative clinical effectiveness, which weighs the benefits and harms / burdens of one treatment option versus another through a systematic review of all available clinical evidence. Feedback from patients and families in addition to input from clinicians, manufacturers, and payers is used to frame the questions that an ICER comparative effectiveness review attempts to answer.
ICER strives to bring these consequential decisions about pricing and access of health interventions out in the open, with patients at the table, where we all can all have an evidence-based discussion about the benefits a new health care intervention provides to patients and their families, and how we should try to align our spending to make sure we get the most health we can out of the dollars available.
ICER Through the Years
2006 – 2010: Developing our Methods
As a research program at Harvard Medical School, Steven D. Pearson founded ICER in 2006 as an organization within Massachusetts General Hospital (MGH).
Dr. Pearson’s goal was more ethical than economic: to identify all the difficult tradeoffs that the US health system was making due to limited resources, and to improve those decisions through transparency, real discussions about the outcomes that matter most to patients, and an independent analysis of the best available clinical evidence.
Throughout these early years, ICER studied how other countries managed the process of health technology assessment (HTA), and we developed analysis methods – for example, a robust evidence rating matrix — that were uniquely suited for the uniquely American health care system. Many of our early assessments focused on devices and hospital interventions, such as proton beam therapy for adult and pediatric cancers, and cardiovascular evaluations like coronary computed tomographic angiography.
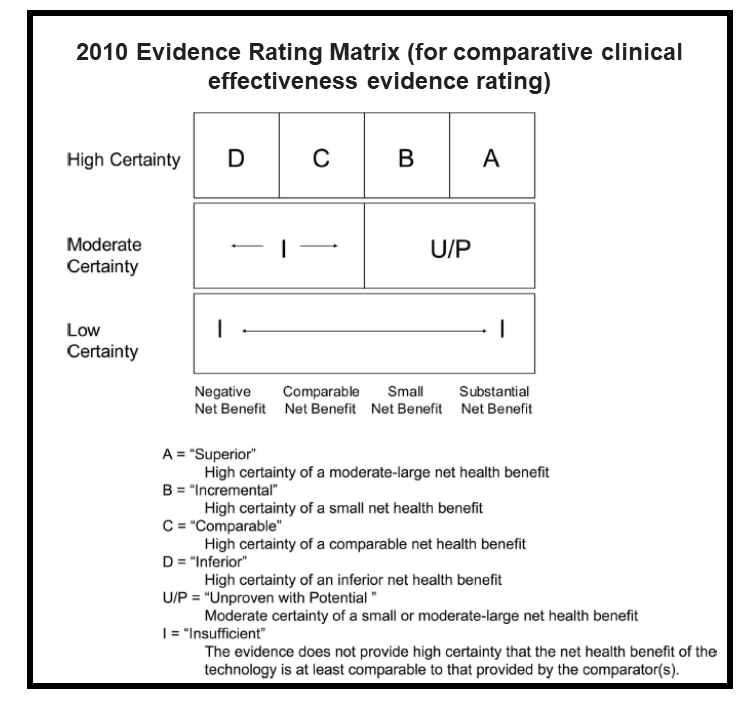
2010 – 2013: Introducing Independent, Public Deliberations
In 2010, ICER transitioned from writing siloed academic analyses to engaging broadly with the US health care system when we established the New England Comparative Effectiveness Public Advisory Council (New England CEPAC), a group of independent experts who would review ICER’s analysis of the evidence, hear patient testimony, and deliberate over the clinical and economic value of health interventions. Shortly after the establishment of the New England CEPAC, we acquired the California Technology Assessment Forum (CTAF) as an additional regional appraisal committee. By bringing these important discussions out into the open, where patients and other US health care stakeholders could inspect the evidence and wrestle with aspects of value and the difficult tradeoffs inherent to any health system, ICER began to be noticed as an organization that could facilitate better health care decision-making across the country. In 2013, ICER spun out of MGH and became an independent, non-profit, nonpartisan, research organization.

2014 – 2016: Gaining National Prominence
The launch of Sovaldi® – the highly effective hepatitis C therapy that cost $1,000 per day – raised national awareness around the life-changing benefits of certain pharmaceuticals, as well as the ever-escalating prices attached to those breakthroughs. A key touchpoint within this national debate was ICER’s 2014 hepatitis C assessment, which helped underscore to media, legislators, and the broader public that pharmaceuticals do not operate as a rational market in the US, and that independent analyses and more transparency can enable better health care decisions, as well as better health outcomes for American patients. As ICER gained national prominence during this period, the Laura and John Arnold Foundation (now known as Arnold Ventures) provided a transformational financial grant that enabled us to further expand the scale and impact of our work.

2017 – 2019: Informing US Policies that Lead to Fair Pricing and Fair Access
Major public and private payers—including state Medicaid agencies, the Department of Veteran’s Affairs, more than 75% of private insurers and PBMs, and multiple employer coalitions—now use ICER’s assessments to inform formulary decisions, coverage criteria and price negotiations.
ICER’s first annual Unsupported Price Increases report found that, in 2017 and 2018, seven of the costliest US drug price hikes had no new important evidence to support their increases; the net price increases on these seven drugs alone cost American payers and patients an additional $4.8 billion over those two years. The National Academy for State Health Policy has drafted a model bill through which state legislatures can increase transparency around this price increases and authorize the state’s tax assessor to recoup a major portion of the additional drug spend.

2020 – 2023: Expanding Patient-Centered Methods & Standardizing Ways for Health Systems to Implement ICER’s Work
Reflecting on the importance of the patient voice, in 2020 we doubled-down on our commitment to give patients a seat at the table during key conversations around fair drug pricing and access. We launched a new patient engagement program where we have committed to 1) offering to help patient organizations prepare for ICER assessments; 2) using what matters most to patients as the North Star of ICER assessments; 3) translating patient input into our economic models; 4) getting patient feedback on the assessment as it progresses; 5) having patients at the head table throughout public deliberation; and 6) partnering with patient organizations after the completion of an ICER assessment to encourage the broader adoption of key policy recommendations by the FDA.
Shortly after the announcement of our 2020 Value Assessment Framework, which promised to expand use of real-world evidence (RWE) throughout our reports, ICER and Aetion announced a partnership and platform to generate decision-grade RWE. ICER hopes that this partnership will set new standards for how RWE can better inform the consequential decisions pharmaceutical companies and health insurers make around drug pricing and patient access.
In November 2020 ICER formally launched ICER Analytics™, a new cloud-based platform that will revolutionize the ability of payers, life science companies, patient groups, and others to develop formularies, negotiate drug prices, and explore new ways to apply evidence in a drive toward a health system that can achieve fair prices and fair access for all.

2024 and Beyond: Pricing in a Pandemic and Passage of the Inflation Reduction Act
After the historic passage of the Inflation Reduction Act in 2022, then-ICER President Steve Pearson, MD, MSc penned an op-ed on the Inflation Reduction Act, and highlighted the next frontier for policymakers — launch prices: “Managing tradeoffs of this nature is an inescapable part of any health system, and I argue that the Inflation Reduction Act is indeed a major victory in that it marks progress in managing the potential tradeoffs between affordability and future innovation more openly and honestly… what is inescapable is that by turning down the spigot of profits for some drugs later in their lifespan, drug companies will react by ramping up the initial launch prices of these (and other) drugs when they first enter the market. Launch prices have already been accelerating rapidly in recent years. A recent study out of the Program on Regulation, Therapeutics, and Law at Brigham and Women’s Hospital in Boston found that median launch prices for new drugs, after rebates to insurers, increased from $1,376 in 2008 to $159,042 in 2021. Reuters reported in mid-August that for drugs launched so far in 2022, the median price is $257,000 for a year’s course. That is nearly four times the annual salary of the average American family — and these expensive drugs must often be taken with others.”
Finally, ICER released updates to our Value Assessment Framework in 2023. We continued to focus on ways to evaluate demographic diversity of clinical trials and expanding our patient engagement program.
ICER Case Studies
Interested in joining our team?
Our team is full of smart, hard-working, and passionate people, and we are looking for more of them to join us. We offer a fast-paced and intellectual atmosphere that is fueled by our mission to make a positive impact on the future of healthcare.
