– Leaders of three health technology assessment organizations convene working group and steering committee to critically and independently research new health economic methods –
BOSTON, February 12, 2025 – The USA-based Institute for Clinical and Economic Review (ICER), England’s National Institute for Health and Care Excellence (NICE), and Canada’s Drug Agency (CDA-AMC) announced the formation of the Health Economics Methods Advisory (HEMA) initiative. The goal of HEMA is to critically and independently research some of the most pressing topics in global health economics and health technology assessment (HTA) methods.
The HEMA initiative brings together an independent working group comprised of research experts, academics, and methodologists in health economics HTA that have both theoretical and practical experience in supporting HTA. The HEMA working group will examine issues that are expected to include topics that are related to health economic methods. HEMA’s selection and prioritization of topics will be guided by a separate steering committee, with representation from the patient, payer, and life sciences communities across all three countries.
ICER’s Chief Scientific Officer and Director of Health Technology Assessment Methods and Engagement, Dan Ollendorf, PhD, MPH stated, “Through this international collaboration, we are looking forward to examining the benefits and disadvantages of new methods in health economics and HTA. There is a need for independent research that can not only critically examine these topics but also assess their feasibility and practicality in HTA settings.” Nick Crabb, Chief Scientific Officer at NICE, and Nicole Mittmann, Chief Scientist at Canada’s Drug Agency, note their agreement on the importance of this aligned work.
HEMA will focus on accomplishing three main goals:
- Examine selected topics — Through the working group and topic specific experts, HEMA will explore topics that include potential benefits, disadvantages, and uncertainties associated with methods, and couple this review with empirical investigation and worked examples where appropriate.
- Provide guidance and recommendations to the HTA community — this guidance may relate to the adoption of novel methods, modifications that might be required, uncertainties in the application of certain methods, and suggestions for further research.
- Coordinate the development of publications — these may include white papers, peer-reviewed articles, workshops, and webinars that focus on the conceptual and empirical applications of alternative methods, assess their applicability and feasibility in HTA settings, and share research and policy perspectives with a broad set of HTA stakeholders.
The University of York’s Mark Sculpher, PhD, Professor of Health Economics and Director of the Centre for Health Economics, will chair the HEMA working group. Dr. Sculpher stated, “HEMA includes methodologists, patients, industry, policy experts, and researchers dedicated to improving our understanding of alternative methods use to assess the cost-effectiveness of interventions to support decision-making processes within the context of HTA. This initiative intentionally includes individuals with a diversity of research experience, viewpoints, and geographic locations across UK, Canada, and US. We look forward to the prospect of having our work inform guidance that the international HTA community can integrate and adapt to their local context.”
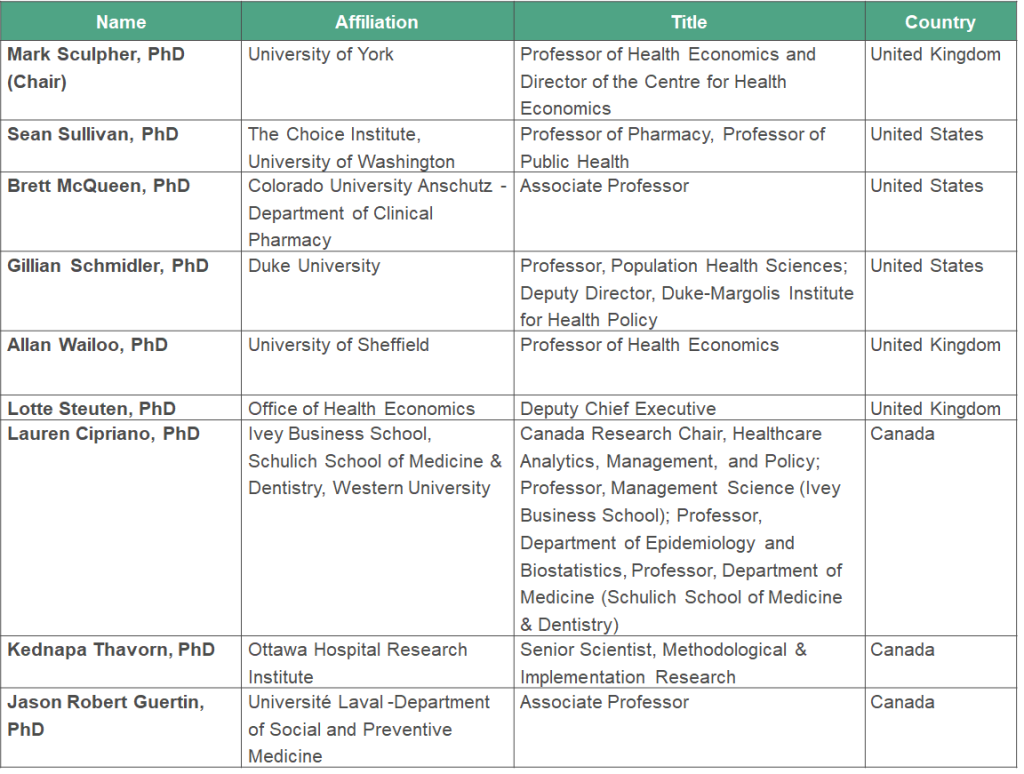
Members of the Working Group include:
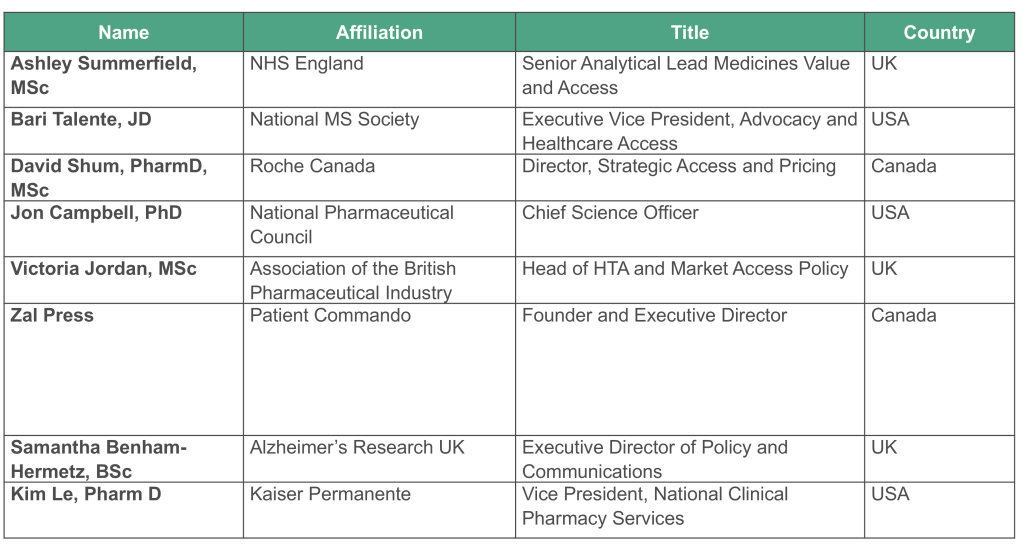
The Steering Committee will include:
About ICER
The Institute for Clinical and Economic Review (ICER) is an independent, non-profit research institute that conducts evidence-based reviews of health care interventions, including prescription drugs, other treatments, and diagnostic tests. In collaboration with patients, clinical experts, and other key stakeholders, ICER analyzes the available evidence on the benefits and risks of these interventions to measure their value and suggest fair prices. ICER also regularly reports on the barriers to care for patients and recommends solutions to ensure fair access to prescription drugs. For more information about ICER, please visit www.icer.org.
About NICE
NICE helps practitioners and commissioners get the best care to people, fast, while ensuring value for the taxpayer. We do this by:
- producing useful and usable guidance for health and care practitioners
- providing rigorous, independent assessment of complex evidence for new health technologies
- developing recommendations that focus on what matters most and drive innovation into the hands of health and care practitioners
- encouraging the uptake of best practice to improve outcomes for everyone.
About Canada’s Drug Agency
Canada’s Drug Agency is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs and medical devices in our health care systems.
