— Report tailored to reflect legislative specifications in the Inflation Reduction Act and subsequent CMS guidance regarding the information that CMS will use to determine initial offers and pursue price negotiation on selected drugs —
BOSTON, October 2, 2023 – The Institute for Clinical and Economic Review (ICER) today posted a Special Report evaluating the evidence on apixaban (Eliquis®, Bristol-Myers Squibb) and rivaroxaban (Xarelto®, Bayer) for the treatment of nonvalvular atrial fibrillation (NVAF).
Downloads: Final Report | Supplement
“The US is beginning a new era in which the federal government will engage in negotiating drug pricing,” said ICER’s President Steven Pearson, MD. “In this task, CMS faces the challenge of evaluating a large evidence base and of integrating considerations regarding unmet need and other factors to arrive at price targets for negotiation. ICER has submitted a Special Report to CMS on two of the drugs whose prices will be negotiated as part of the public comment process defined in CMS guidance. This ICER report includes sections on multiple elements related to value, providing different options for translating evidence into initial offer prices and for assessing counter-offers from drug makers. We recognize that our report will be one of many inputs CMS may consider, and we hope that it will help them as they build a reliable and transparent drug price negotiation process on behalf of the American people.”
Key Clinical Findings
ICER selected NVAF as the indication in which to evaluate evidence given that the great majority of prescriptions for both drugs are for this condition. Unmet need was evaluated through both qualitative perspectives informed by interviews with patients and clinical experts, and by quantitative estimates of health “shortfalls” experienced by individuals with NVAF.
To estimate the comparative therapeutic impact of Eliquis and Xarelto in NVAF, ICER compared each drug to both warfarin and dabigatran, finding in summary that:
- For Eliquis, there is high certainty of a small net benefit compared with warfarin (B rating), andmoderate certainty of a comparable or small net benefit compared with dabigatran (C+ rating).
- For Xarelto, there is high certainty of a small net benefit compared with warfarin (B rating), and high certainty of a comparable net benefit compared with dabigatran (C rating).
Comparative Effectiveness and Cost Findings
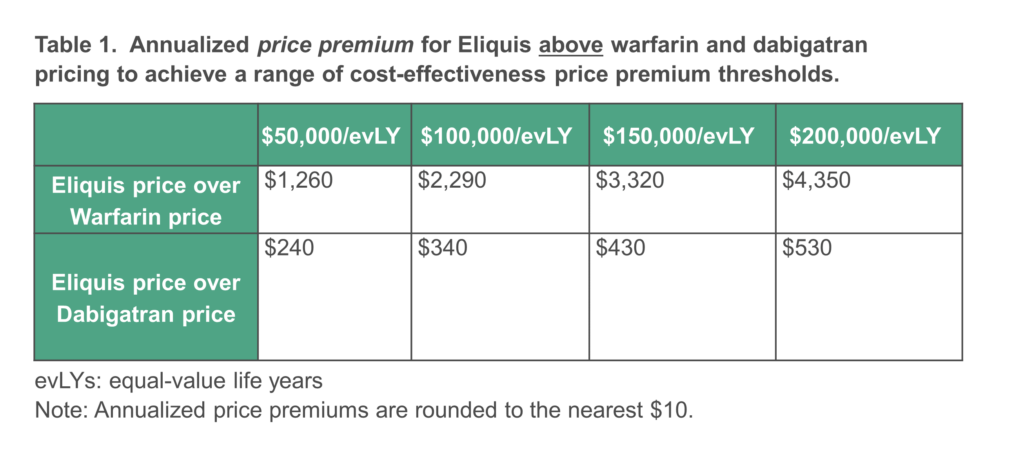
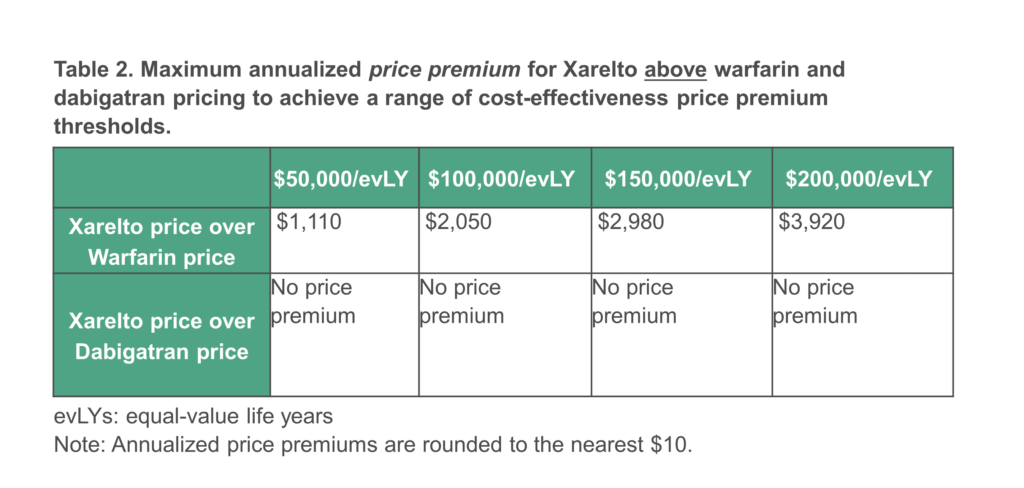
Modeling the relative advantages of Eliquis and Xarelto over warfarin in stroke prevention, myocardial infarction prevention, major bleeding episodes, and other patient-centered outcomes, current evidence supports a substantial price premium for Eliquis and Xarelto above warfarin. Comparing all these outcomes for Eliquis and Xarelto to dabigatran, however, our modeling suggests a smaller price premium for Eliquis and no price premium for Xarelto.
In keeping with CMS guidance, our modeling did not make any use of the quality-adjusted life year (QALY), and instead relied on the equal value life year (evLY), an alternative measure that values all life-extending treatment effects equally for all patients, regardless of pre-existing disability or age. Different potential price targets based on modeling the overall comparative clinical effectiveness of Eliquis and Xarelto versus the two comparator drugs are shown in the Tables below. All prices shown are price premiums above the current comparator price paid by CMS for warfarin or dabigatran, respectively. ICER did not attempt to determine the current prices being paid by CMS.
About ICER
The Institute for Clinical and Economic Review (ICER) is an independent non-profit research institute that produces reports analyzing the evidence on the effectiveness and value of drugs and other medical services. ICER’s reports include evidence-based calculations of prices for new drugs that accurately reflect the degree of improvement expected in long-term patient outcomes, while also highlighting price levels that might contribute to unaffordable short-term cost growth for the overall health care system.
