–Report finds alemtuzumab represents best long-term cost-effectiveness; prices for most drugs not well-aligned with added value for patients. Stakeholder collaboration needed to improve access for patients.–
Boston, Mass., January 26, 2017 – The Institute for Clinical and Economic Review (ICER) has released an Evidence Report assessing the comparative clinical effectiveness and value of 15 disease-modifying therapies (DMTs) for treatment of relapsing-remitting and primary-progressive multiple sclerosis. This Evidence Report will be the subject of the upcoming public meeting of the California Technology Assessment Forum (CTAF) on February 16, 2017.
The analyses showed that when compared to supportive care, all of the DMTs under review improved health outcomes related to delayed disability and prevention of relapses. The DMTs showed mixed results in how well they worked compared to generic glatiramer acetate, a commonly used treatment in current practice. While evidence on clinical effectiveness demonstrated improvements on key outcomes, cost-effectiveness analyses showed that current prices for nearly all drugs reviewed are not aligned with the long-term added value for patients.
“Evidence suggests that all of these drugs can play a significant role in delaying disability and preventing relapse in MS, two factors that we know are of the highest importance to patients. But, survey results included in the report show that patients are often limited in their treatment choices due to financial and coverage barriers,” noted ICER President Steven D. Pearson, MD, MSc. “Our goal is to spur discussion among stakeholders to ensure that patients have access to the medications at prices that are aligned with the value they bring to patients.”
In addition to clinical and economic analyses, the results of which are outlined below, the report also includes findings from discussions with patient groups, as well as a survey conducted by the MS Coalition to highlight patient values in treatment decisions. Among the top priorities of patients were the need to delay disability and prevent relapses, followed closely by the desire to continue working or participating in their normal activities. Despite the clinical benefit of many of the drugs reviewed, many patients reported that their treatment choices were influenced by their ability to afford the out-of-pocket costs associated with treatment, which can often be very high and make some treatments inaccessible to patients, along with barriers to access due to health plan coverage of treatments.
The ICER report reviewed the following agents:
Injectable Agents
- Daclizumab (Zinbryta®, Biogen/AbbVie)
- Glatiramer acetate (Copaxone®, Teva)
- Glatiramer acetate (Glatopa®, Sandoz (Novartis))
- Interferon beta-1a (Avonex®, Biogen)
- Interferon beta-1b (Betaseron®, Bayer)
- Interferon beta-1b (Extavia®, Novartis)
- Interferon beta-1a (Rebif®, EMD Serono)
- Peginterferon beta-1a (Plegridy®, Biogen)
Oral Agents
- Dimethyl fumarate (Tecfidera®, Biogen)
- Fingolimod (Gilenya®, Novartis)
- Teriflunomide (Aubagio®, Sanofi-Genzyme)
Infused Agents
- Alemtuzumab (Lemtrada®, Sanofi-Genzyme)
- Natalizumab (Tysabri®, Biogen)
- Ocrelizumab (Ocrevus®, Roche/Genentech)
- Rituximab (Rituxan®, Roche/Genentech)
Rituximab has not been approved by the FDA for use in MS.
Key Findings of the Evidence Report
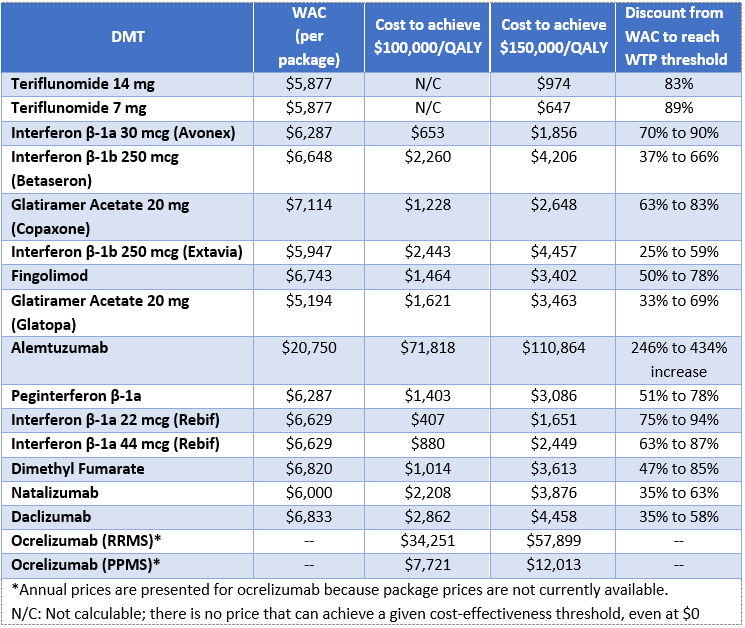
Findings from clinical trials indicated that, in regards to delay of disability and prevention of relapse, there is high certainty of at least a small net health benefit for each of the drugs reviewed when compared to supportive care. The efficacy of the drugs, however, comes at a high relative cost. Economic analyses show that the costs of the therapies at current levels are not aligned with their long-term value. Cost-effectiveness thresholds for most drugs substantially exceeded the commonly accepted $100,000-$150,000 per quality-adjusted life year threshold, even when using prices that account for typical discounts and rebates that may apply in real-world practice. Alemtuzumab had the best estimated cost-effectiveness at $34,659 per QALY when compared to supportive care, though this drug may only be suitable for a subset of patients due to its safety profile. ICER applied an average discount to the wholesale acquisition cost (WAC) for each drug class to arrive at an estimated net price for use in the cost-effectiveness analyses. The drug discounts were determined from information on net pricing provided by SSR Health. The full report includes more information on the cost-effectiveness ratios, including calculations that use WAC, as well as more detail on the methods and results of the entire comparative clinical effectiveness and comparative value analyses.
The report also calculates value-based price benchmarks that help to identify the prices at which the cost of the drugs align with the benefit they bring to patients. ICER’s analyses found that the prices of a majority of the drugs reviewed would need to be discounted anywhere from 25% to 94%, depending on the drug, in order to meet commonly accepted thresholds for cost-effectiveness. Alemtuzumab, however, would remain cost-effective even with an increase in price. By highlighting prices that better align with the benefit the drugs bring to patients, ICER hopes to prompt stakeholder collaboration to develop strategies to ensure that manufacturers are rewarded for their innovative research while patients gain access to the treatments that best meet their needs.
ICER is committed to a transparent public engagement process to ensure that all stakeholders have the opportunity to provide input to the reports and public meetings. After ICER’s Draft Evidence Report was released on November 22nd, interested stakeholders had a four-week period to provide comments. ICER staff considered these comments and made revisions to the report as needed. The Evidence Report, as well as the accompanying voting questions, public comments, and ICER’s written response to comments, are available on the ICER website.
About ICER
The Institute for Clinical and Economic Review (ICER) is an independent non-profit research institute that produces reports analyzing the evidence on the effectiveness and value of drugs and other medical services. ICER’s reports include evidence-based calculations of prices for new drugs that accurately reflect the degree of improvement expected in long-term patient outcomes, while also highlighting price levels that might contribute to unaffordable short-term cost growth for the overall health care system.
ICER’s reports incorporate extensive input from all stakeholders and are the subject of public hearings through three core programs: the California Technology Assessment Forum (CTAF), the Midwest Comparative Effectiveness Public Advisory Council (Midwest CEPAC), and the New England Comparative Effectiveness Public Advisory Council (New England CEPAC). These independent panels review ICER’s reports at public meetings to deliberate on the evidence and develop recommendations for how patients, clinicians, insurers, and policymakers can improve the quality and value of health care. For more information about ICER, please visit ICER’s website.
Appendix
This table represents prices for each drug that would achieve cost-effectiveness thresholds ranging from $50,000 to $150,000 per QALY gained, along with the wholesale acquisition cost per package. It was not possible to calculate a threshold price for all DMTs at the lower thresholds. This was because even if the price of the DMT were $0, the patient still accrued costs from second-line drugs and other care. As those other costs are particularly high relative to supportive care, it was not possible to decrease the WAC enough to reach the threshold.
Value-based price benchmarks for MS treatments