Health technology assessment (HTA) is an established practice, conducted by many agencies and research organizations worldwide. Some variation in methods across organizations is necessary — it reflects local preferences and priorities. There is also significant value in evaluating new methods as an international community before they are considered for local implementation.
The Health Economics Methods Advisory (HEMA) group has been convened by the leaders of three HTA organizations to critically and independently examine and assess new methods and processes:
- The USA-based Institute for Clinical and Economic Review (ICER);
- Canada’s Drug Agency (CDA-AMC);
- England’s National Institute for Health and Care Excellence (NICE).
ICER’s Chief Scientific Officer and Director of Health Technology Assessment Methods and Engagement Dan Ollendorf, PhD, MPH will coordinate this effort in partnership with leaders at NICE and CDA.
For any questions please reach out to Liis Shea, Senior Program Director, at [email protected].

HEMA aims to:
- Examine selected topics — Through the working group and topic specific experts, HEMA will explore topics that include potential benefits, disadvantages, and uncertainties associated with methods, and couple this review with empirical investigation and worked examples where appropriate.
- Provide guidance and recommendations to the HTA community — this guidance may relate to the adoption of novel methods, modifications that might be required, uncertainties in the application of certain methods, and suggestions for further research.
- Coordinate the development of publications — these may include white papers, peer-reviewed articles, workshops, and webinars that focus on the conceptual and empirical applications of alternative methods, assess their applicability and feasibility in HTA settings, and share research and policy perspectives with a broad set of HTA stakeholders.
To learn more about the HEMA Working Group Principles, click here.
Sarah K. Emond, ICER’s President and CEO, MPP stated:
“Since our inception, ICER has been committed to methods development. We re-examine our Value Assessment Framework every few years, convene a Methods Advisory Group several times per year, and solicit feedback on our analyses and processes from stakeholders across the health system. Through HEMA, we hope to engage regularly with the international HTA community to discuss the advantages and drawbacks of new methods. And while implementation will vary across regions, this is an opportunity for the broader health economics community to critically examine and potentially pilot the feasibility of new approaches to HTA.”
HEMA will undertake independent assessments of some of the most pressing health economics methods topics, such as considerations of dynamic efficiency and dynamic pricing in economic models, use of novel or nontraditional value elements in cost-effectiveness analysis, integration of health equity considerations in quantitative analysis and deliberation, and more. The working group will include representatives from each of the three countries, with diversity in expertise and viewpoint. HEMA’s selection and prioritization of topics will also be guided by a separate Steering Committee, with representation from the patient, payer, and life sciences communities.
Visit this page regularly to receive updates on HEMA and Steering Committee membership as well as initial topics. In addition, as HEMA begins publishing new papers on HTA methods, we will include fully-accessible links to those documents and other dissemination vehicles below.
For any questions please reach out to Liis Shea, Senior Program Director, at [email protected].
HEMA Working Group Members:

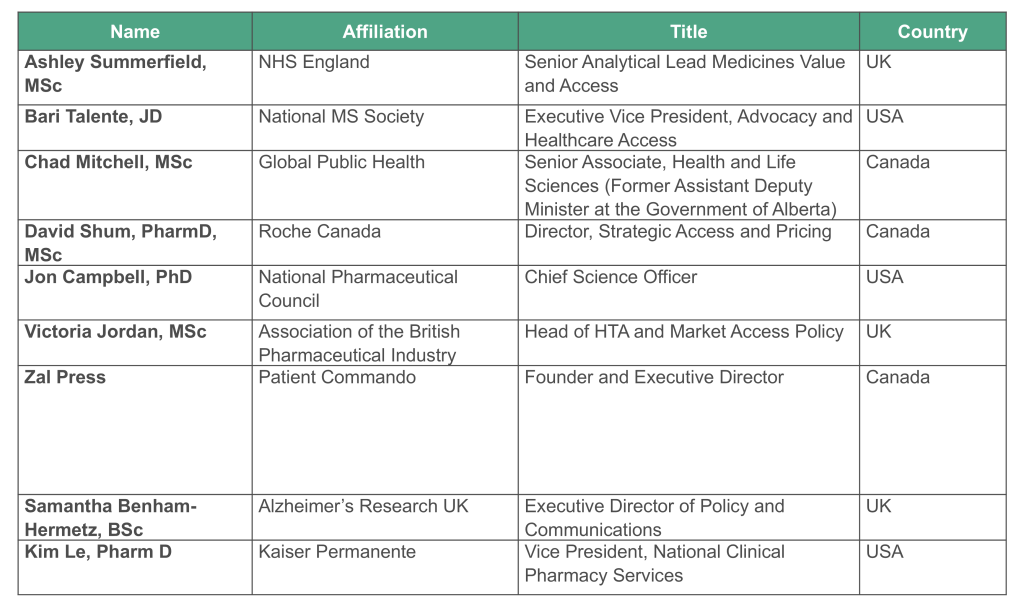
HEMA Steering Committee Members:
Related Press Releases
Health Economics Methods Advisory Group Selects First Area for Study: Assessing Treatment Benefits Appropriate to Consider in HTA Decision-Making
The Health Economics Methods Advisory (HEMA) group will conduct an assessment of the benefits of treatment that are appropriate to consider in health technology assessment.
03/18/2025ICER, NICE, and Canada’s Drug Agency Convene the Health Economics Methods Advisory
Leaders of three health technology assessment organizations convene working group and steering committee to critically and independently research new health economic methods.
02/12/2025